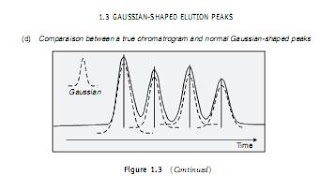
This equation is based on a Gaussian distribution, similar to that of plate theory. Its simplified form, proposed by Van Deemter in 1956, is well known (expression 1.30). The expression links the plate high H to the average linear velocity of the mobile phase ¯u in the column (Figure 1.11).
The three experimental basic coefficients A, B and C are related to diverse physico-chemical parameters of the column and to the experimental conditions. If H is expressed in cm, A will also be in cm, B in cm2/s and C in s (where velocity is measured in cm/s).
This equation reveals that there exists an optimal flow rate for each column, corresponding to the minimum of H, which predicts the curve described by Equation 1.30.
The loss in efficiency as the flow rate increases is obvious, and represents what occurs when an attempt is made to rush the chromatographic separation by increasing the pressure upon the mobile phase.
However, intuition can hardly predict the loss in efficiency that occurs when the flow rate is too slow. To explain this phenomenon, the origins of the terms A, B and C must be recalled . Each of these parameters represents a domain of influence which can be perceived on the graph (Figure 1.11).
The curve that represents the Van Deemter equation is a hyperbola which goes through a minimum H
min
when:
Term A is related to the flow profile of the mobile phase passing through the stationary phase. The size of the particles (diameter d
), their dimensional distribution and the uniformity of the packing (factor characteristic of packing ) can all be the origin of flow paths of different length which cause broadening of the solute band and improper exchanges between the two phases. This results in turbulent or Eddy diffusion, considered to have little importance in liquid chromatography and absent for WCOT capillary columns in GC (Golay’s equation without term A, cf. paragraph 1.10.2). For a given column, nothing can be done to reduce the A term.