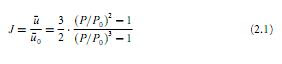The mobile phase is a gas (helium, hydrogen or nitrogen), either drawn from a commercially available gas cylinder or obtained, in the case of hydrogen or nitrogen, from an on-site generator, which provides gas of very high purity. The carrier gas must be free of all traces of hydrocarbons, water vapour and
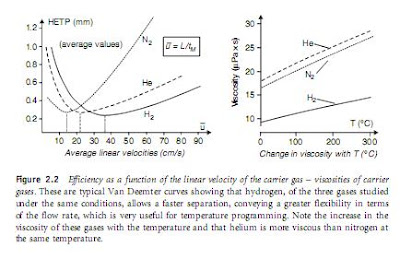
oxygen, because all of these may deteriorate polar stationary phases or reduce the sensitivity of detectors. For these reasons the carrier gas system includes filters containing a molecular sieve to remove water and a reducing agent for other impurities. The nature of the carrier gas has no significant influence upon the values of the partition coefficients K of the compounds between the stationary and mobile phases, owing to an absence of interaction between the gas and solutes. By contrast, the viscosity of the carrier gas and its flow rate have an effect on the analytes’ dispersion in the stationary phase and on their diffusion in the mobile phase (cf. Van Deemter’s equation), and by consequence upon the efficiency N and the sensitivity of detection (Figure 2.2). Hydrogen is the carrier gas of choice. If this gas cannot be used for safety reasons, helium may be substituted.
The pressure at the head of the column (several tens to hundreds of kPa) is stabilized either mechanically or through an electronic pressure control (EPC) in order that the flow rate remains constant at its optimal value. This device is valuable because if the analysis is performed with temperature programming, the viscosity of the stationary phase and by consequence the loss of charge in the column, increase with temperature. Therefore to maintain the carrier gas flow constant, the pressure must be finely tuned to compensate this effect. The result is a faster analysis and a longer life for the column.
The injector and the detector have dead volumes (hold-up volumes) which are counted in the total retention volume. In GC, since the mobile phase is a gas, the flow rate measured at the outlet of the column should be corrected by a compression factor J, which compensates for the higher pressure at the head of the column (cf. expression 2.1).
If a chromatogram contains a peak for a compound that is not retained on the stationary phase, it is possible to calculate the average linear velocity of the progression, ¯u, of the carrier gas. Elsewhere by installing a flow meter at the outlet of the instrument (atmospheric pressure P) and knowing the diameter of the column the velocity ¯u00 of the carrier gas, at the end of the column, can be deduced. The ratio between these two velocities is equal to J, the compression factor, which is linked to the relative pressure P/P
The injector and the detector have dead volumes (hold-up volumes) which are counted in the total retention volume. In GC, since the mobile phase is a gas, the flow rate measured at the outlet of the column should be corrected by a compression factor J, which compensates for the higher pressure at the head of the column (cf. expression 2.1).
If a chromatogram contains a peak for a compound that is not retained on the stationary phase, it is possible to calculate the average linear velocity of the progression, ¯u, of the carrier gas. Elsewhere by installing a flow meter at the outlet of the instrument (atmospheric pressure P) and knowing the diameter of the column the velocity ¯u00 of the carrier gas, at the end of the column, can be deduced. The ratio between these two velocities is equal to J, the compression factor, which is linked to the relative pressure P/P