Analytical chromatography is used essentially in quantitative analysis. In order to achieve this effectively, the areas under the peaks must be determined with precision, which in turn necessitates well-separated analytes to be analysed. A certain experience in chromatography is required when the analysis has to be optimized, employing all available resources in terms of apparatus and software that can simulate the results of temperature modifications, phases and other physical parameters.
In gas phase chromatography, the separations can be so complex that it can be difficult to determine in advance whether the temperature should be increased or decreased. The choice of column, its length, its diameter, the stationary phase composition and the phase ratio (VM/VS) as well as the parameters of separation (temperature and flow rate), are amongst the factors which interact with each other.
In gas phase chromatography, the separations can be so complex that it can be difficult to determine in advance whether the temperature should be increased or decreased. The choice of column, its length, its diameter, the stationary phase composition and the phase ratio (VM/VS) as well as the parameters of separation (temperature and flow rate), are amongst the factors which interact with each other.
The resolution and the elution time are the two most important dependent variables to consider. In all optimizations, the goal is to achieve a sufficiently complete separation of the compounds of interest in the minimum time, though it should not be forgotten that time will be required to readjust the column to the initial conditions to be ready for the next analysis. Chromatography corresponds, in fact, to a slow type of analysis. If the resolution is very good then optimization consists to save time in the analysis. This can be done by the choice of a shorter column – recalling that the resolution varies with the square root of the column length (cf. the parameter N of formula 1.28 and Figure 1.10).
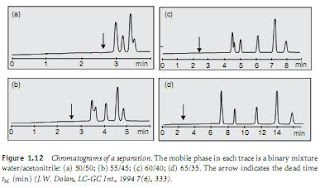
Figure 1.12 shows the optimization of a separation, by liquid chromatography, of a mixture of aromatic hydrocarbons. In this case, optimization of the separation has been carried out by successive modifications of the composition of the mobile phase. Note that by optimizing the sequence in this manner, the cycle time of analysis increases.
If only certain compounds present in a mixture are of interest, then a selective detector can be used which would detect only the desired components. Alternately, at the other extreme, attempts might be made to separate the largest number of compounds possible within the mixture.
Depending upon the different forms of chromatography, optimization can be more or less rapid. In gas phase chromatography optimization is easier to achieve than in liquid chromatography in which the composition of mobile phase must be considered: software now exists that can help in the choice of mobile phase composition. Based upon certain hypotheses (Gaussian peaks), the areas of poorly defined peaks can be found.
The chromatographer must work within the limits bound by a triangle whose vertices correspond to three parameters which are in opposition: the resolution, the speed and the capacity (Figure 1.13). An optimized analytical separation uses the full potential of the selectivity which is the most efficient parameter. In the chromatographer’s triangle shown, the optimized conditions are close to the vertex of resolution.
If only certain compounds present in a mixture are of interest, then a selective detector can be used which would detect only the desired components. Alternately, at the other extreme, attempts might be made to separate the largest number of compounds possible within the mixture.
Depending upon the different forms of chromatography, optimization can be more or less rapid. In gas phase chromatography optimization is easier to achieve than in liquid chromatography in which the composition of mobile phase must be considered: software now exists that can help in the choice of mobile phase composition. Based upon certain hypotheses (Gaussian peaks), the areas of poorly defined peaks can be found.
The chromatographer must work within the limits bound by a triangle whose vertices correspond to three parameters which are in opposition: the resolution, the speed and the capacity (Figure 1.13). An optimized analytical separation uses the full potential of the selectivity which is the most efficient parameter. In the chromatographer’s triangle shown, the optimized conditions are close to the vertex of resolution.